Deep Cycle Battery for Boat
About the deep cycle battery for boat and how to choose and install. Boat service or house power loads require a battery that can withstand many cycles of long continuous discharge, and repeated recharging. This deep cycling requires the use of the suitably named deep cycle battery. First, we will go through some basic battery chemistry to refresh on why a deep cycle boat battery is the battery of choice for any boat, in particular those that cruise and are away from the comfort of a battery charger in a marine.
Deep Cycle Boat Batteries Basics
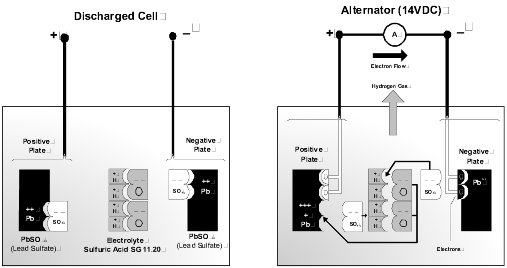
Lead Acid Batteries. The fundamental theory of the battery is that a voltage is developed between two electrodes of dissimilar metal when they are immersed in an electrolyte. In the typical lead-acid cell, the generated voltage is 2.1 volts. The typical 12-volt battery consists of 6 cells, which are internally connected in series to make up the battery.
Cell Components. The principal cell components are:
1. Lead Dioxide (PbO2) — the positive plate active material.
2. Sponge Lead (Pb) — the negative plate material.
3. Sulfuric Acid (H2SO4) — the electrolyte.
Deep Cycle Battery for Boat Cycles
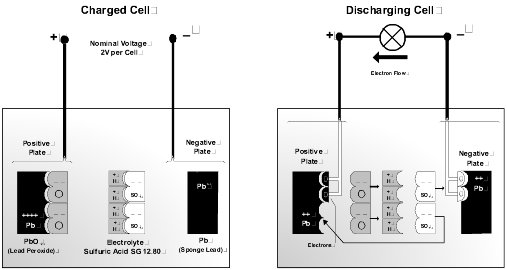
Discharge Cycle. Discharging of the battery occurs when an external load is connected across the positive and negative terminals. A chemical reaction takes place between the two plate materials and the electrolyte. During the discharge reaction, the plates interact with the electrolyte to form lead sulfate and water. This reaction dilutes the electrolyte, reducing the density. As both plates become similar in composition, the cell loses the ability to generate a voltage.
Charge Cycle. Charging simply reverses this reaction. The water decomposes to release hydrogen and oxygen. The two plate materials are reconstituted to the original material. When the plates are fully restored, and the electrolyte is returned to the nominal density the battery is completely recharged.
Deep Cycle Battery for Boat Electrolytes
Battery Electrolyte. The cell electrolyte is a dilute solution of sulfuric acid and pure water. Specific gravity (SG) is a measurement defining electrolyte acid concentration. A fully charged cell has an SG typically in the range 1.240 to 1.280, corrected for temperature. This is an approximate volume ratio of acid to water of 1:3. Pure sulfuric acid has an SG of 1.835, and water a nominal 1.0. The following factors apply to battery electrolytes.
Temperature Effects. For accuracy, all hydrometer readings should be corrected for temperature. Ideally, actual cell temperatures should be used, but in practice ambient battery temperatures are sufficient. Hydrometer floats have the reference temperature printed on them and this should be used for calculations. As a guide, the following should be used for calculation purposes.
1. For every 1.5°C the cell temperature is above the reference value, add 1 point (0.001) to the hydrometer reading.
2. For every 1.5°C the cell temperature is below the reference value, subtract 1 point (0.001) from the hydrometer reading.
Nominal Electrolyte Densities. Recommended densities are normally obtainable from battery manufacturers. In tropical areas it is common to have battery suppliers put in a milder electrolyte density, which does not deteriorate the separators and grids as quickly electrolytes for temperate climate.
Battery Water. When topping up the cell electrolyte, always use distilled or de-ionized water. Rainwater is acceptable, but under no circumstances use tap water. Tap water generally has an excessive mineral content or other impurities that may pollute and damage the cells. Impurities introduced into the cell will remain, and concentrations will accumulate at each top up, reducing service life. Long and reliable service life is essential so the correct water must always be used. Water purity levels are defined in various national standards.
Deep Cycle Battery for Boats Sulfation
Plate Sulfation. Sulfation is the single greatest cause of battery failure, and occurs as follows:
During discharge, the chemical reaction causes both plates to convert to lead sulfate. If recharging is not carried out within a couple of hours, the lead sulfate starts to harden and crystallize. This is characterized by white crystals on the typically brown plates and is almost non-reversible. If a battery is only 80% charged, this does not mean that only 20% is sulfating, the entire plate material has not fully converted and subsequently sulfates.
The immediate effect of sulfation is partial and permanent loss of capacity as the active materials are reduced. Electrolyte density also partially decreases, as the chemical reaction during charging cannot be fully reversed. This sulfated material introduces higher resistances within the cell and inhibits charging. As the level of sulfated material increases, the cell's ability to retain a charge is reduced and the battery fails. The deep cycle battery has unfairly gained a bad reputation, but the battery is not the cause, improper and inadequate charging is. As long as some charging is taking place, even from a small solar panel, a chemical reaction is taking place and sulfation will not occur.
Efficiency. Battery efficiency is affected
by temperature. At 0°C, efficiency falls by 60%. Batteries in warm tropical
climates are more efficient, but may have reduced life spans, and batteries
commissioned in tropical areas often have lower acid densities. Batteries in
cold climates have increased operating lives, but are less efficient.
Self Discharge. During charging, a small quantity of antimony or other impurities dissolve out of the positive plates and deposit on the negative ones. Other impurities are introduced with impure topping up water and deposit on the plates. A localized chemical reaction then takes place, slowly discharging the cell. Self-discharge rates are affected by temperature, with the following results:
1. At 0°C, discharge rates are minimal.
2. At 30°C, self-discharge rates are high and the specific gravity can decrease by as much as 0.002 per day, typically up to 4% per month.
Check out the Marine Electrical and Electronics Bible, 4th Edition for all you needs to know about batteries used for deep cycle house loads.
Deep Cycle Battery for Boat Construction
The battery is typified by the use of thick, high-density flat-pasted plates, or a combination of flat and tubular plates. The plate materials may also contain small proportions of antimony to help stiffen them. Porous and insulating separators are used between the plates. Glass matting is used to assist in retaining active material on the plates. Plate material can break away as plates expand and contract during charge and recharge cycles. If material accumulates at the cell base, a cell short circuit may occur, although this is less common in modern batteries. If material is lost the plates will have reduced capacity or insufficient active material to sustain the chemical reaction with resultant cell failure. Much has be done to develop stronger and more efficient plates. Rolls have their Rezistox positive plates. The grid design has fewer heavier sections to hold the high density active material. This is due to the dynamic forces that normally cause expansion and contraction with subsequent warping and cracking. Separator design has also evolved and Rolls use double insulated thick glass woven ones that totally encase the positive plate along with a microporous polyethylene envelope. This retains any material shed from the plates than cause cell short circuits.
Deep Cycle Battery for Boat Cycling
Cycling. The number of available cycles varies between individual battery makes and models. Typically, it is within the range of 800–1500 cycles of discharge to 50% of nominal capacity and complete recharging. Battery life is a function of the number of cycles and the depth of cycling. Batteries discharged to only 70% of capacity will last appreciably longer than those discharged to 40% of capacity. In practice you should plan your system so that discharge is limited to 50% of battery capacity. The typical life of batteries where batteries are properly recharged and cycle capabilities maximized can be up to 5–10 years. Deep Cycle Battery for Boat and the Boat Battery, essential to safety and security afloat.