Gel Batteries for Boats
Gel batteries for boats are not like normal lead-acid cells as the gel cell has a solidified thixotropic gel as an electrolyte, which is locked into each group of plates. The gel electrolyte has a high viscosity and during the charge and discharge process develops voids and cracks. These can impede the flow of acid and cause capacity loss. During charging the gel also liquifies due to its thixotropic properties. Solidification after charging can exceed an hour as thixotropic gels have a reduced viscosity under stress. The newer types use phosphoric acid in the gel to retard the sulfation hardening rates. Water loss can occur in VRLA batteries, and the oxygen recombination cycle is used to minimize electrolyte loss. Sources of water loss are small for each source, however cumulatively they can cause failure, and the process is called dry-out. Water loss causes include reduced recombination efficiency, high charge voltages and corrosion of the positive grid. Transpiration through the cell casing also can occur, typically in temperatures exceeding 40°C. Batteries should be installed in cool areas wherever possible. For more detailed information go to the Marine Electrical and Electronics Bible.
The 4th Edition of the Marine Electrical Electronics Bible Get your copy and start becoming self sufficient and save money on expensive technician callouts. The 4th Edition of the Marine Electrical Electronics Bible Get your copy and start becoming self sufficient and save money on expensive technician callouts.Gel Batteries for Boats
Construction. The plates are reinforced with calcium, rather than antimony, which reduces self-discharge rates, and they are relatively thin. This facilitates gel diffusion and improves the charge acceptance rate as diffusion problems are reduced. The separator provides electrical and mechanical isolation of the plates, and must have a high porosity to facilitate ion migration and electrolyte acceptance. Each cell has a safety valve to relieve excess pressure if the set internal pressure is exceeded. Typical values are 114 psi (100mbar). The valve recloses tightly to prevent oxygen from entering the cell. Lead-acid batteries suffer from corrosion of the current collector grid. Typical grids in VRLA batteries are manufactured from lead-calcium-tin, lead-tin-alloys or lead-antimony-cadmium. Positive plate grids corrode due to the conversion of lead to lead dioxide. This corrosion effect doubles for every 10˚C, as well as charge voltages and electrolyte density. As the lead dioxide requires a substantially higher volume, this causes mechanical stressing and deformation of the grid. The effects can cause capacity loss, internal short circuits and even cell case ruptures, which increases with length of service. The process consumes water reducing the electrolyte, and causes lowered performance. VLRA cells are also prone to chemical degradation of the negative plate lugs and strap surfaces and this is called sulfate rot. This is caused by the oxygen recombination reaction and electrolyte inorganic sulfate salts.
Gel Batteries for Boats
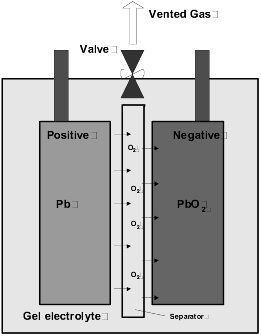
Gel Cell Charging. Gel batteries for boats have a much higher charge acceptance rate, and therefore a more rapid charge rate is possible. A gel cell cannot tolerate having any equalizing charge applied and this overcharge condition will seriously damage them. The illustration shows the oxygen recombination cycle.
During charging the current causes decomposition of the water and the evolvement of oxygen at the positive plate. The oxygen diffuses through the unfilled glass mat separator pores to the negative plate, and chemically reacts to form lead oxide, lead sulfate and water. The charge current then reduces and does not evolve hydrogen gas. The end of charge voltage is typically 2.22 to 2.28 Vpc (volts per cell). If recombination of hydrogen is incomplete during overcharge conditions, the gases may vent to the battery locker causing an explosion risk. Although accepting a higher charge rate than a lead-acid deep cycle battery, and consequentially charging to a higher value, there is at a certain point the problem of attaining full charge, and therefore capacity usage of the battery bank.
Gel Batteries for Boats
As no fast charge devices can be safely used, a longer engine run time is required for complete recharging. While these batteries will accept some 30-40% greater current than an equivalent lead-acid battery, they are restricted in the voltage levels allowed, so you cannot use any fast charging system. Typical open circuit voltages are 100% charge is 12.85–12.95 volts, 75% is 12.65 volts, 50% is 12.35 volts, 25% is 12 volts and 11.8 volts is flat. Gel batteries for boats are intolerant to over voltage charge conditions and can be damaged in over charge situations. The normal optimum voltage tolerance on Dryfit units is 14.4 volts. Check out Gel Battery technical details for Sonnenschein.
There are some minimal heating effects during charging, and this is caused by the recombination reaction. Where batteries are not kept within reasonable temperature ranges thermal runaway can occur. This normally occurs during charging when the temperature of the battery and charge current create a cumulative increase in temperatures and leads to battery destruction. Continuous over or undercharging of gel cells is the most common cause of premature failure. In many cases this is due to use of imprecise automotive type chargers.
Gel Batteries for Boats
Gel batteries for boats do however have a much greater cycling capability than normal starting batteries, and in many applications they are ideal. If the safety valves malfunction the cells are also easily damaged by oxygen contamination, although in a quality gel cell battery this is uncommon.
A quality deep cycle lead acid battery can have a life exceeding 2,500 cycles of charge and discharge to 50%. A gel cell has a life of approximately 800–1,000 cycles. They do have a much greater cycling capability than normal starting batteries, but not of quality deep cycle or AGM batteries.
All About the gel batteries for boats and about boat batteries.